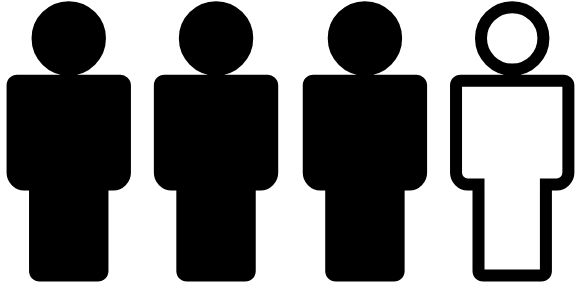
Research suggests that approximately one in four people with APDS face early mortality by the age of 30 years old.8
40%
of patients have a family history of IEIs.
In almost 40% of cases, patients have a family history of IEIs,9 however APDS can present spontaneously with neither parent being a carrier of the genetic variants which cause the disease.10 It is estimated that APDS affects one to two people per million, including both adults and children.11